In an era that demands sustainable energy solutions, efficient and eco-friendly energy storage and conversion are crucial. Hydrogen, with high energy density and zero emissions, is promising but faces storage, transport, and distribution challenges. The PeCATHS project partners work to overcome these hurdles.
This research, published in ACS Sustainable Chemistry & Engineering, presents a new approach to hydrogen storage, promoting sustainable chemistry. Titled “Electrohydrogenation of Benzonitrile into Benzylamine under Mild Aqueous Conditions,” it advances Liquid Organic Hydrogen Carriers (LOHCs), enabling safe and efficient hydrogen storage in a liquid. The innovation supports greener, milder aqueous processes, demonstrating the commitment of PeCATHS partners to tackling environmental and energy challenges for a sustainable future.
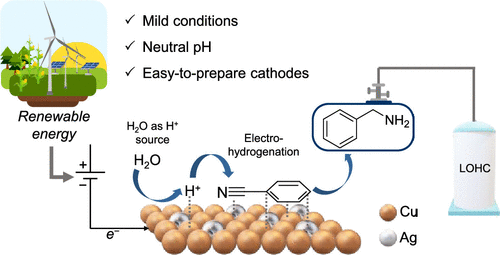
Hydrogen’s appeal as a clean energy carrier is undeniable, but its gaseous nature presents logistical challenges. Storing it as compressed gas requires high energy, safety measures, and complex infrastructure. Liquid Organic Hydrogen Carriers (LOHCs) offer a promising alternative: organic compounds that chemically store and release hydrogen through reversible reactions, providing safer, more energy-dense, and transportable storage using existing liquid fuel infrastructure.
Hydrogenation of organic molecules like nitriles usually uses thermocatalytic methods with high temperatures, pressures, hydrogen, and additives. While effective, these conditions are energy-intensive, risky, and can create unwanted by-products. Nitrile reduction is tough because of the stable carbon-nitrogen triple bond, needing harsh conditions. This reliance on demanding conditions makes sustainable synthesis difficult.
The research by the PeCATHS partners offers a compelling electrochemical alternative for the hydrogenation of nitriles. Their study focuses on the electrohydrogenation of benzonitrile (BZN) into benzylamine (BZA), a valuable building block for various chemical industries. Crucially, the BZN/BZA pair also serves as a promising Liquid Organic Hydrogen Carrier (LOHC).
This innovation uses electrochemistry, which utilises electrical energy to drive reactions at mild conditions like room temperature and atmospheric pressure, often using water. This method is safer, more energy-efficient, and aligns with the principles of green chemistry.
PeCATHS partners’ success is supported by carefully selecting and optimising electrode materials. They studied pure copper (CuE) and copper-silver (CuEAg) electrodes. CuE electrodes are made by electrodepositing a copper layer on copper foil, forming a ‘cauliflower-like’ porous structure that boosts surface area.
To understand the reaction mechanism and confirm the proton source, researchers used deuterated water (D2O). Nuclear Magnetic Resonance (NMR) experiments confirmed that water acts as the proton source for nitrile hydrogenation. This highlights a major advantage over conventional techniques, which require external hydrogen gas and hazardous reagents, making the process safer and more environmentally friendly.
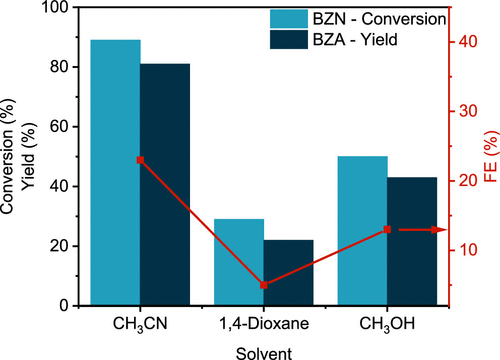
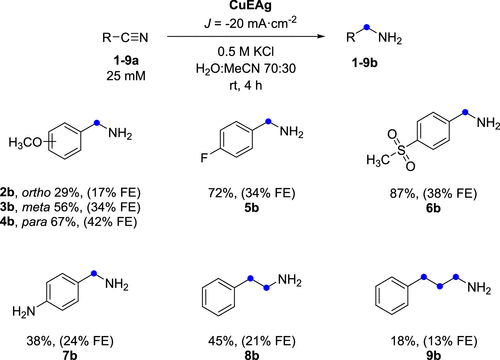
The versatility of the copper-silver electrode was investigated for various aromatic nitriles. While some substituted nitriles showed lower yields, the study demonstrated the broad applicability of their method to a range of nitrile compounds, underscoring its potential for diverse chemical syntheses.
The electrohydrogenation method developed by the PeCATHS partners stands as a testament to the principles of green chemistry, offering a significantly more environmentally friendly alternative to traditional thermal catalysis.
Despite challenges like low benzonitrile solubility and high overpotentials, this electrochemical method offers advantages. It avoids high-pressure hydrogen, improves safety, and enables precise control. Localised reactions near tailored electrodes can boost selectivity. Promising yields and FEs under mild conditions suggest that further optimisation can overcome mass transfer limitations, enabling broader use.
This research offers a safer, eco-friendly method for nitrile reduction and advances Liquid Organic Hydrogen Carriers (LOHCs). By using water as a proton source under mild conditions, the PeCATHS team highlights electrochemistry’s potential to transform industrial processes. It exemplifies how interdisciplinary research and innovative materials can create practical, scalable, and sustainable solutions for energy and chemical challenges.
As we move towards a renewable-powered future, efficient and safe hydrogen storage and transport will be vital. PeCATHS partners’ advancements bring us closer to this goal, promising a greener, sustainable chemical industry.
Read the article published in ACS Sustainable Chemistry & Engineering: https://pubs.acs.org/doi/10.1021/acssuschemeng.5c02168